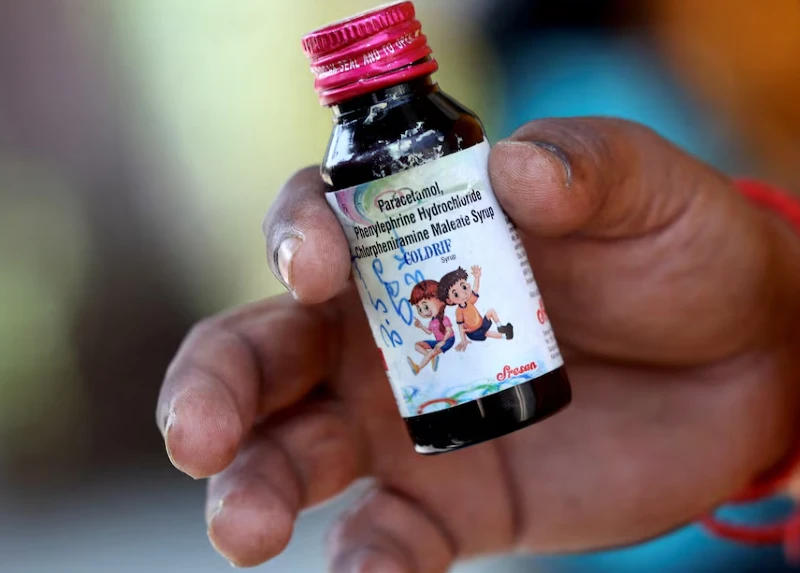
Seven out of nine children in MP’s Chhindwara district died due to poisonous cough syrup as traces of diethylene glycol (DEG) were found in them. The test in Tamil Nadu confirmed the presence of DEG in the syrup, leading to its ban by the Madhya Pradesh government. Along with the cough syrup, other medicines manufactured by Sresan Pharmaceuticals, a Tamil Nadu-based company, have also been banned in the state.
Sources suggested that the seven children who died had consumed the same syrup. The confirmation report was issued by the Director of Drugs Control, Tamil Nadu, on 4th October. The report confirmed the presence of 48.6% diethylene glycol in Coldrif and declared it “Not of Standard Quality”. The substance is extremely harmful to the kidneys and leads to death on ingestion. It is used in antifreeze and brake fluid manufacturing.
After receiving the report, Chief Minister of Madhya Pradesh Mohan Yadav banned the sale, purchase, and distribution of coldrif across the state, effective immediately. The Food and Drug Administration has also asked all drug inspectors to immediately seize all the remaining stocks of the drug and inspect other batches.
The FDA directive has asked every officer to ensure that the syrup is not sold in their respective jurisdiction and that all available products should be sent for inspection. Inspectors have also been directed to inspect other products of Sresan and send them for lab tests.
The first instance of the case started in late August in Parasia, a village near Chhindwara, where children developed kidney disorders after experiencing mild fever and cough. It was later found that the seven kids who died due to similar circumstances had ingested the syrup. The reports from Tamil Nadu finally confirmed the source of poison as the manufacturer itself.
Cases of syrup poisoning have occurred even before, and show a lack of strict compliance on the part of the manufacturers. Legal actions and accountability need to be restored to prevent such cases in future, as it risks several lives.